Abstract
Introduction:
Adult T-cell leukemia-lymphoma (ATLL) is caused by HTLV-1, and carries a dismal prognosis urging the development of new therapies. CD30 expression has been reported at variable frequencies in ATLL. The anti-CD30 monomethyl-auristatin-E conjugate brentuximab vedotin (BV), can be an effective treatment option for Hodgkin lymphoma, anaplastic T-cell lymphomas, and various peripheral T-cell lymphoma types. However, the impact role of BV in ATLL has not been established, which warrants an in-depth characterization of CD30 expression and clinical investigation of BV in this disease.
Methods:
ATLL specimens from 107 patients (pts) were classified according to Shimoyama criteria (A=acute, C=chronic, L=lymphomatous, and S=smoldering) and analyzed for CD30 expression. Unfavorable chronic (UC) had elevated LDH level < twice the normal value. Cases were confirmed or analyzed by immunohistochemistry at our institution using anti-human CD30 antibody (Dako) on formalin-fixed paraffin-embedded tissue or cellblock sections prepared from cytospun CD4+-enriched leukemic cells. The cutoff positive value was 20%. Outcomes were compared according to prior treatment. Soluble(s) CD30 levels were measured in sera of 21 pts by ELISA. Low-passage ATLL cell lines (ATLL-84c and ATLL-97c established from pts with A-type) were treated with BV, and apoptosis was measured by annexin V and FACS analysis. Five pts with relapsed CD30+ ATLL (L-type=3, A-type=2) were treated with BV.
Results:
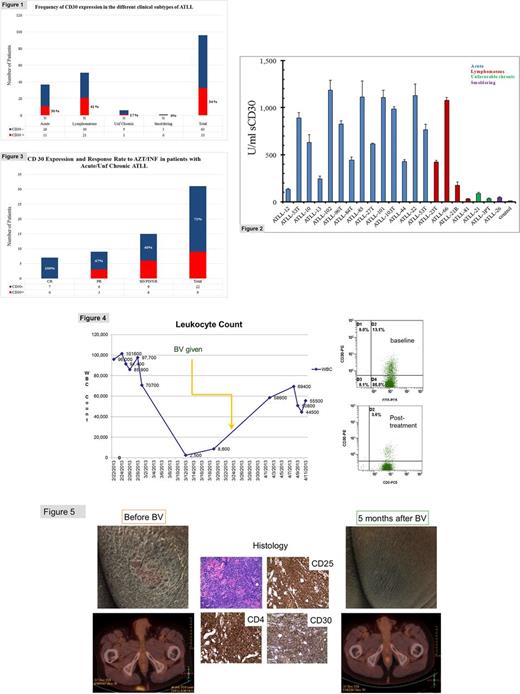
ATLL specimens obtained from 95 (L=51, A=37, UC=6, and S=1) untreated pts, and 13 (L=7, and A=6) pre-treated patients were analyzed. The percent frequencies of CD30+ cases within untreated subtypes were L=41%, A=30%, UC=17%, and S=0%, as compared to L=14% and A=17% in the pre-treated group (Figure 1). There was a trend towards a higher frequency of CD30 expression in L-type vs. A-type (p=0.37, two-sided Fisher's test). Sera analyzed from 21 pts revealed positive CD30 levels regardless of CD30tumor status, suggesting ATLL cells may shed the receptor (Figure 2). The overall response (OR) and complete response (CR) rates in A/UC-type pts (n=31) treated with AZT (zidovudine) plus interferon-α (AI) were 59% (13/22) and 32% (7/22) in the CD30- group (n=22), compared to 33% (3/9) and 0% in CD30+ group (n=9) respectively (Figure 3). BV had no significant clinical significant impact in 4 out of 5 (80%) CD30+ treated pts (2 L and 2 A). A transient hematologic response was observed in one CD30+ A-type patient, followed by leukemic progression with CD30- cells (Figure 4). By contrast, BV induced a sustained CR lasting 2 years in an L-type patient with diffuse homogenously CD30+ expression (Figure 5). In vitro treatment of patient-derived ATLL cell lines confirmed BV induced more apoptosis in cells expressing higher CD30 (ATLL-84c) as compared to cells low in CD30 expression (ATLL97c).
Conclusions:
CD30 expression in ATLL is heterogeneous and occurs at variable frequencies, and tends to be higher in L-type, and lower in A/UC-types that respond to AZT-interferon therapy. Our data suggest CD30 may represent an elusive target in A-type ATLL, thus warranting further optimization of BV in leukemic subtypes. The analysis of additional cases is still ongoing and the final results will be presented at the meeting.
This project has been accomplished with the support of "ASH HONORS award" achieved in 2016.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal